What is metabolism?
Our holistic wellbeing is deeply reliant on our metabolic health. Let’s explore the function of our metabolism before we look at metabolic health in relation to our brain specifically.
Metabolism is defined as all chemical reactions involved in maintaining living cells and organisms. In other words, metabolism involves the processing of the energy your body needs to breathe, circulate blood, grow, repair cells, and everything else it does to survive.
Metabolism is further divided into two categories:
Catabolism: The breakdown of molecules to obtain energy.
Anabolism: The synthesis of all compounds needed by the cells.
What is metabolic health?
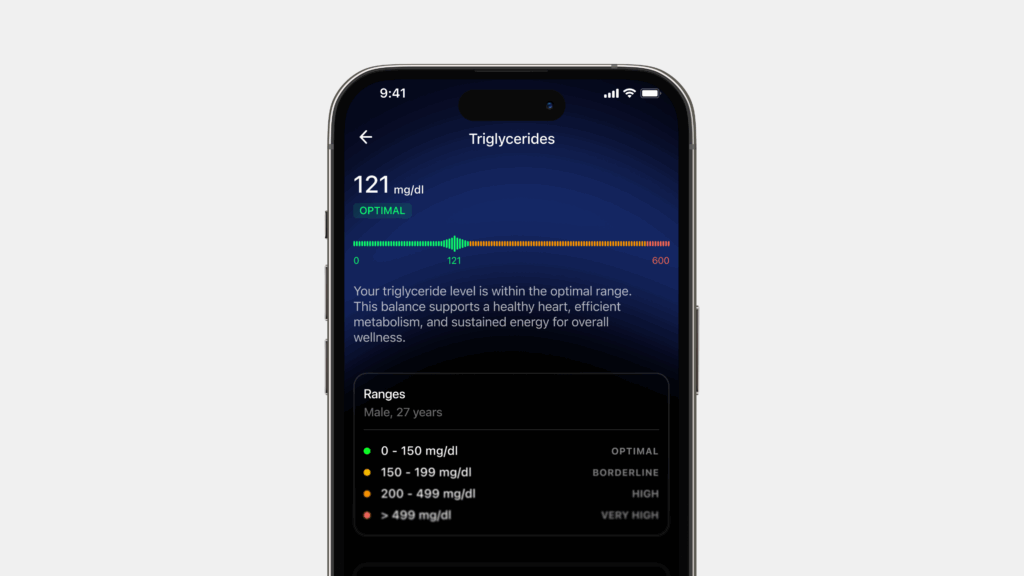
Metabolic health is described as having ideal levels of blood sugar, triglycerides, high-density lipoprotein (HDL) cholesterol, blood pressure, and waist circumference, without using medications.
How Does the Brain Regulate Metabolism?
Glucose is the fuel of the brain. The human brain needs a constant flow of glucose for our cells to function. Although our brain is less than 2% of our body weight, it needs up to 20% of its glucose to produce energy. Glucose is critical for cellular maintenance and also helps in creating adenosine triphosphate (ATP), an energy transferring molecule that stores energy and releases it as fuel for cellular activity.
Insulin is a hormone released by the pancreas. It regulates glucose in the body. Insulin is responsible for managing protein, carbohydrate metabolism, and helping cellular growth and division.
When we eat, insulin levels in our body increase to absorb the sugar spike in our blood and store it for energy. This allows glucose levels in our bloodstream to decline. The pancreas then produces glucagon, a hormone that prompts the liver to release stored sugar. The interaction between glucagon and blood sugar is vital for stable blood glucose levels in the brain and body.
However, with age, the risk of insulin resistance increases.
The cells of individuals who have insulin resistance don’t respond well to insulin, barring glucose from entering them with ease. The glucose level in their blood rises over time, even as their body produces more insulin and the cells resolutely resist it. This risk is associated with senile skeletal muscle dysfunction. Skeletal muscles are tissues that play a crucial role in the body’s glucose metabolism. After a mixed meal, skeletal muscles are involved in glucose disposal and have an influence on insulin sensitivity. As we age, the skeletal muscles go through many changes and dysfunctions.
In more recent studies, the hypothalamus is emerging as the primary role player in homeostatic regulation of glucose in our bodies. Our brains can translate many metabolic signals through the food we eat, insulin secretion, energy expenditure, hepatic (liver) glucose production and glucose/fatty acid metabolism in adipose tissue (body fat) and skeletal muscle. Efficient communication between the brain and peripheral metabolic organs is critical for the maintenance of energy and glucose homeostasis (the balance of insulin and glucagon to maintain blood glucose).
The way the brain controls the production of glucose in the liver and moderates it has a major impact on the body and the way it perceives memory and cognition. Research suggests that neurons involved in energy homeostasis are situated on the satiety and hunger networks. Insulin in the brain also influences neuroprotective effects (which may cause recovery or regeneration of the nervous system).
A study suggests that the ageing of the brain is a consequence of the decline of energy metabolism. Glucose metabolism in the nerves decreases as we age, resulting in lower energy. The drastic dearth of nicotinamide adenine dinucleotide (NAD), a coenzyme whose levels determine the speed of the ageing process, plays an important role in this correlation. The study makes a case for a focus on energy metabolism and rectification of NAD deficiency in neurons for future treatment designs.
Insulin resistance can result in cognitive decline. This is why metabolic health is increasingly important in determining cognitive ability as we age since blood sugar levels are an important marker of metabolic health.
It’s widely held that the gut is the second brain. The gut-brain axis (GBA) comprises bidirectional exchange between the central and the enteric nervous systems via the vagus nerve, connecting emotional and cognitive centres of the brain with peripheral intestinal functions. Gut microbiota modulates this interaction. The microbes, particularly the bacteria, that are in a pocket in the large intestine are referred to as our gut microbiome. A healthy gut is important because it produces chemicals that help make neurotransmitters; for example, serotonin, an antidepressant neurotransmitter, is made in the gut. Research suggests that gut microbiota influence memory, mood, cognition, sleep and stress reactivity.
Cognitive Decline and Brain Health
WHO defines brain health as an emerging and growing concept that encompasses neural development, plasticity, functioning, and recovery across the life course.
All life ages as time goes by, and a natural outcome of the ageing process is the shrinking of the brain. Cognitive decline is the ageing of neurons and a decrease in the speed at which the brain functions. This is very different from cognitive impairment, which can happen because of external factors such as an accident.
Since everyone ages, everyone will experience some amount of cognitive decline. Although everybody is unique, there is evidence that high-quality nutrition (a diet that’s high-fibre, rich in fruits and vegetables and unsaturated fats) and regular exercise are a critical part of keeping this decline to a minimum. Improving brain plasticity is an important part of maintaining brain health. This means stimulating our brain cells to create new connections. There is also evidence that keeping our minds active can help in generating new brain cells that can protect us from future loss of cells. Puzzles, wordplays, riddles, taking a new class are some of the ways you can increase brain plasticity.
New studies suggest that there is a correlation between insulin resistance and cognitive decline in individuals with Alzheimer’s and other forms of dementia (an umbrella term for diseases that feature a host of progressive cognitive decline symptoms). Recent studies are examining the role of ketones in substituting glucose to avert rapid deterioration of cognitive health. Brain glucose uptake is disrupted in Alzheimer’s. Ketones are produced in our liver when glucose and insulin in our bodies are low. For example, your body might produce ketones when you fast for hours without any food or beverage. More studies are needed to see if glucose can be effectively substituted to prevent cognitive decline.
An important factor in brain health and cognitive ability is also the impact of stress and how much we sleep. Stress elevates the hormone cortisol in our body, and studies show that cortisol can impact the quality of our sleep. Poor quality of sleep and sleep deprivation is closely associated with the impairment of the hypothalamic-pituitary–adrenal (HPA) axis, leading to hyperactivation. Sleep and stress share many pathways that influence the central nervous system (CNS) and metabolism, and they possibly comprise mechanisms that partially shape the increasing prevalence of metabolic disorders. Sleep-disordered breathing can lead to nocturnal awakening, associated with cortisol release and autonomic nervous system activation. It can also lead to HPA axis activation.
Oxidative stress, inflammation and glycation are important factors that determine how doctors and scientists will look at the future of metabolic health and the brain.

What is Oxidative stress?
Oxidative stress is defined as a disturbance in the balance between the production of reactive oxygen species (free radicals) and antioxidant defences. Free radicals fight pathogens that provoke infections. They are extremely reactive, while antioxidants stabilize the reactive nature of free radicals, creating harmony.
A reactive oxygen species (ROS) is a molecule containing oxygen that has gained an extra electron. This causes the molecule to become highly chemically reactive. These free radicals are produced during normal cell metabolism. ROS accumulation leads to dysfunction in the neurons.
In the course of oxidative stress, a surplus of free radicals can be detrimental to structures inside brain cells and even lead to cell death, which may increase the risk of Parkinson’s disease. A low-fat diet, accompanied by antioxidants–rich fruits and vegetables, whole grain, and foods such as fish and nuts that are repositories of brain-healthy omega-3 fatty acids, can help prevent oxidative stress.
Studies show that free radicals are associated with cognitive decline, neurodegenerative disorders and cardiovascular health, among others. High intake of sugar creates too many free radicals which can result in hypoxia (lower levels of cellular oxygen). When the brain suffers from lack of cellular oxygen, it can lead to inflammation of the brain and other cellular metabolic processes critical to our brain health.
Inflammation and cognitive decline
A key feature of most metabolic disorders is inflammation. Brain inflammation can be a result of inflammation in the body in response to infections, leaky gut or an unmanaged autoimmune condition. This can activate a flooding of pro-inflammatory chemicals (cytokines), released from fat cells, in the body.
Infections and injuries prompt the body’s immune response. Immune cells, called macrophages, generate free radicals while warding off germs. These free radicals can ravage healthy cells, leading to inflammation. Generally, inflammation fades away after the infection dissipates, but oxidative stress can also activate an inflammatory response.
Inflammation in the brain marrs brain cells, speeds up ageing and causes atrophy of the brain.
A study shows that chronic low-grade inflammation is linked with visceral obesity. Visceral fat accumulates in the abdominal area near vital organs like our liver and is linked with many health disorders, including insulin resistance, a marker of metabolic health dysfunction.
Studies suggest that there is a notable interaction with inflammation and metabolic syndrome on cognitive decline.
Exercise, especially HIIT workouts (High-Intensity Interval Training), is known to increase brain cell growth and reduce inflammation. HIIT helps in releasing BDNF (brain-derived neurotrophic factor), a protein that helps protect nerve cells. There is evidence that interval training can stop cellular ageing by pumping up the production of proteins for the mitochondria, the powerhouse of the cell that generates energy for cellular activity, which usually starts to deteriorate with time.
A healthy diet, low in sugar and high in fibre, combined with consistent learning to increase brain plasticity, are integral parts of brain metabolic health.
What is Glycation?
When excess sugars attach themselves to the proteins and lipids in our bodies, a build-up occurs which is called AGEs (advanced glycation end products). Studies show that the plaque build-up of AGEs can be more detrimental to brain health than regular plaque build up. This study illustrates how the chronic accumulation of AGEs progresses with aging. AGEs can build up in vascular tissues which stiffen and thicken vascular walls. This can increase the risk of hypertension, a marker of metabolic health.
Metabolic Syndrome
Metabolic syndrome is defined as an interconnected group of physiological, biochemical, clinical and metabolic factors that directly increase the risk of cardiovascular disease, type 2 diabetes mellitus (T2DM) and mortality. <2.> Research suggests that metabolic syndrome presents risks for the nervous tissue and threatens neuronal function. It may make one susceptible to neurological disorders.
Conclusion
Our brain health is dynamic and vital to our metabolic wellbeing. New science indicates that metabolic health is anchored by healthy insulin and glucose levels in our brains. Insulin resistance is linked to cognitive decline. Oxidative stress, glycation, and inflammation can significantly impact our brain health and can cause or exacerbate cognitive decline, obesity, and cardiovascular diseases. Exercise, diet, and learning are reliable tools that can help extend the lifespan of our brain health, and can also help extend our holistic health.
Disclaimer: The contents of this article are for general information and educational purposes only. It neither provides any medical advice nor intends to substitute professional medical opinion on the treatment, diagnosis, prevention or alleviation of any disease, disorder or disability. Always consult with your doctor or qualified healthcare professional about your health condition and/or concerns and before undertaking a new health care regimen including making any dietary or lifestyle changes.
References