Autoimmunity is the presence of self-reactive B cells (autoantibodies) and T lymphocytes that react with the self-antigens of a person. Autoantibodies are a wonderful diagnostic tool to interpret autoimmunity. The essential feature of autoimmune diseases (AIDs) is that tissue injury is caused by the immunologic reaction of the organism against its own tissues (auto-reactive lymphocytes).
A balanced microbiome in the gut is an important aspect to indicate good health as many researchers have proven that intestinal dysbiosis (gut impairment, dysfunction, decreased microbiome diversity and increased inflammation) is hugely interlinked with AIDs such as type 1 diabetes, multiple sclerosis, rheumatoid arthritis and systemic lupus erythematosus.

Highlights
- Current research outcomes,
- Autoimmune in interplay with gut microbiota,
- Approaches to manage debilitating autoimmune diseases.
Current research outcomes
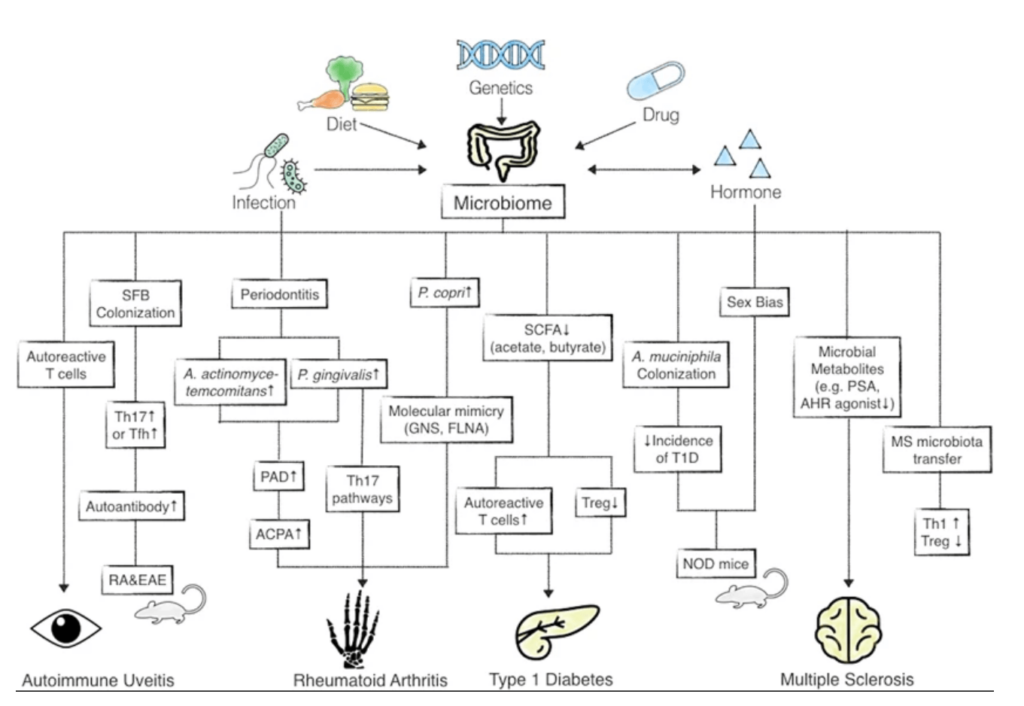
Many research articles have published how gut microbiota can trigger the development of disease and specific commensal microbiota associated with AIDs. In a 2016 study, Lerner et al. revealed the possible mechanisms behind triggering AIDs; this included molecular mimicry (identical peptides), significant T cell activation (bystander activation during infection), amplification of autoimmunity by cytokines (activated by T cells) and defective post-translational modification of proteins (or PTMP) in the lumen of the intestine to generate neo-epitope (vigorous T cell activation).
Autoimmune disorders in interplay with gut microbiota
Under physiological conditions, homeostasis normally exists between the commensal microbiota, the epithelial cells that line the interior of the intestines (referred to as intestinal epithelial cells or IECs) and the immune cells within the tissues. A major study also revealed that microbiota, IECs and immune cells—together termed “supra organism”—are affected by environmental factors (smoking, antibiotics, enteropathogens) as well as lifestyle factors (stress, anxiety, sleep), which vary the gut flora and genetics of the individual host and upset homeostasis during the ageing process. This results in a chronic state of immune dysregulation, leading to the increase of ‘autoimmune’ diseases.
Early-life exposure—is gender a concern?
Yes, women are more inclined to be affected by AIDs than men. A review article emphasizes the importance of understanding early-life microbiome exposure which can invite the risk of several diseases and altered immune and metabolic health. During childbirth, a mother’s genital microbiota, like good bacteria (Lactobacillus spp.) is passed on to the newborn. On the other hand, infants born via C-section are unable to reap these benefits and are consequently at a higher risk of developing dysbiosis and other upper respiratory infections, obesity and AIDs.
The interlink between commensal microbiota and autoimmune disorders
The endogenous commensal microbiota within the intestines, acquired in the early stages from the mother and eventually from the environment around, play a central role in disorder development (pathogenesis). It has been seen that the commensal microbiota in patients accelerates the disease and has been reported in affected and non-afflicted individuals, suggesting a state of dysbiosis (e.g., Proteobacteria spp. and Escherichia coli spp.)
In immune-mediated rheumatic disorders like arthritis, it is common to see individuals with issues of inflammatory bowel disease (IBD), which signifies the role of the gut. A common AID like IBD affects the gastrointestinal (GI) tract in the form of Crohn’s disease and ulcerative colitis. A recent report suggests the importance of diverse bacteria in the gut fastening recovery in IBD patients, indicating a role in the pathogenesis.
The second brain and gut–brain axis
The gut microbiome and enteric nervous system (the second brain) are conjoined with your immune system and associated with the cause of your AID. The two-way signalling between the gut and the brain is referred to as the gut–brain axis (or GBA) and involves pivoting the functions of the brain with the periphery of the intestine by modulating neural, immunological and endocrine mechanisms. The second brain regulates the GI tract and is influenced by the gut microbiome.
Stress and gut leak
In adults, chronic stress affects the composition of the gut microbiota and sees an increase of Bacteroides spp. and Clostridium spp. along with elevated interleukin 6 (or IL-6) levels, indicating immune activation. Chronic stress also makes the gut leaky, increasing circulating levels of endotoxins or liposaccharides (or LPS). Increased gut permeability is observed in patients with multiple sclerosis and Parkinson’s disease.
Gut microbiome test
Gut microbiota is recognized as an effective target for specific therapeutic interventions. Metagenome-wide studies and shotgun sequencing are methods to test AIDs through saliva, oral saliva and faecal samples. Outcomes of these tests enable physicians globally to diagnose and treat patients. A gut microbiome test (stool sample) constitutes an evidence-based diagnosis that will guide you to a better understanding of the diversity and abundance of not just bacteria but fungi and yeast as well, apart from giving an overview of inflammation in the gut which can flare up AIDs.
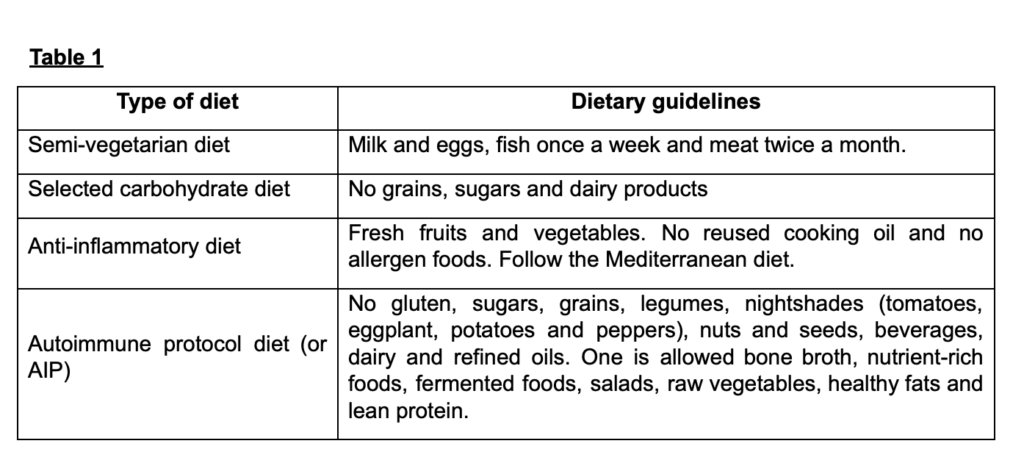
Nutrition therapy
As an adjunctive treatment, various diets have been followed by health consultants to help to reduce inflammation and slow down the progress of AIDs. Here is the overview of the same.
Novel therapeutic targets
Probiotics:
To restore healthy gut flora, bacterial strains like Bifidobacteria spp., Lactobacillus spp., Lactococcus spp., Pediococcus spp. and other non-pathogenic strains of E. coli (Nissle), Streptococcus (salivarius), Saccharomyces (boulardii) are recommended.
Faecal microbiota transplantation:
Many researchers published the pros and cons of faecal microbiota transplantation (or FMT) and strictly recommended its use under the supervision of health practitioners. In this case, health strains from the donor stool are transferred to the host gut to repopulate the healthy microbiome.
Lifestyle modifications
It is observed that managing anxiety, stress and insomnia helps people to recover from AIDs. Some recommended options in this regard include physical activity, guided breathing practices, yoga, regular exercise, being social, being in a less toxic environment, practising deep relaxation, sleeping well (seven to eight hours) and minimizing junk food. These modifications help to reduce the symptoms of AIDs and contribute to overall lifelong well-being.
Conclusion
AIDs are highly influenced by various physiological and environmental factors underlying especially altered gut microbiome. Recent technological advancements facilitate a deeper investigation of the correlation between gut health and AIDs. Lifestyle modifications, early diagnosis and supporting conventional drug therapies with a nutritious diet help to decelerate the progression of AIDs. Further substantial evidence-based studies are needed to understand the role of specific strains in gut flora in the pathogenesis of AIDs.
Disclaimer: The contents of this article are for general information and educational purposes only. It neither provides any medical advice nor intends to substitute professional medical opinion on the treatment, diagnosis, prevention or alleviation of any disease, disorder or disability. Always consult with your doctor or qualified healthcare professional about your health condition and/or concerns and before undertaking a new healthcare regimen including making any dietary or lifestyle changes.
References
- “Definition of Autoimmunity”. Johns Hopkins Hospital, Pathology. https://pathology.jhu.edu/autoimmune/definitions/.
- Wu H. J., Wu E. (2012). “The role of gut microbiota in immune homeostasis and autoimmunity.” Gut Microbes; 3:4–14.
- de Oliveira, Gislane Lelis Vilela et al. (2017). “Intestinal dysbiosis and probiotic applications in autoimmune diseases.” Immunology vol. 152,1: 1–12. doi:10.1111/imm.12765.
- Lerner, A., Aminov, R., and Matthias, T. (2016). “Dysbiosis May Trigger Autoimmune Diseases via Inappropriate Post-Translational Modification of Host Proteins.” Frontiers in Microbiology, 7, 84. https://doi.org/10.3389/fmicb.2016.00084.
- Pott J., Hornef M. (2012). “Innate immune signalling at the intestinal epithelium in homeostasis and disease.” EMBO Rep.;13(8):684–98. doi:10.1038/embor.2012.96.








