INTRODUCTION
The blood sugar levels of all individuals fluctuate and cause tremendous changes in our bodies. Understanding what causes these, specifically in non-diabetic people, can help us improve our metabolic health. In this article, we dive deeper into the causes of high blood glucose levels, why they surge after you eat and what can be done to prevent it.
Before we do that, it’s imperative to understand the different ways in which the hormone, insulin, behaves in the body and the process of glucose metabolism.
Glucose Metabolism and Insulin Action
What is insulin resistance and how does insulin work? Insulin is a hormone created by the pancreas that controls the amount of glucose in a person’s bloodstream at any given moment. It helps store glucose in the liver, fat and muscles, and regulates the body’s metabolism of carbohydrates, fats and proteins. After eating, blood sugar levels rise. Insulin released by the pancreas helps the cells absorb blood sugar for energy and storage. With this absorption, glucose levels in the bloodstream begin to decline. The pancreas then produces glucagon, a hormone that prompts the liver to release stored sugar. This interaction of glucagon and blood sugar ensures stable blood glucose levels in the body and the brain. The cells of individuals who have insulin resistance don’t respond well to insulin, barring glucose from entering them with ease. The glucose level in their blood rises over time even as their body produces more insulin, as the cells resolutely resist insulin.
Circulating glucose is derived from three sources: intestinal absorption during the fed state, glycogenolysis, and gluconeogenesis. The major determinant of how quickly glucose appears in the circulation during the fed state is the rate of gastric emptying. Other sources of circulating glucose are derived chiefly from hepatic processes (processed by the liver): glycogenolysis, the breakdown of glycogen, the polymerized (combined together chemically) storage form of glucose; and gluconeogenesis, the formation of glucose primarily from lactate and amino acids during the fasting state.
Glycogenolysis and gluconeogenesis are partly under the control of glucagon, a hormone produced in the α-cells of the pancreas. During the first 8–12 hours of fasting, glycogenolysis is the primary mechanism by which glucose is made available. Glucagon facilitates this process and thus promotes glucose appearance in the circulation. Over longer periods of fasting, glucose, produced by gluconeogenesis, is released from the liver.
What is Non-Diabetic Hyperglycemia(High Blood Glucose)?
Hyperglycemia without diabetes means having high blood glucose levels (sugar).
This means that the cells in the body are unable to absorb glucose efficiently, despite the secretion of insulin by the pancreas. Hyperglycemia that occurs in people without diabetes is referred to as non-diabetic hyperglycemia.
An illness or injury can cause hyperglycemia suddenly. A chronic disease may be the cause of hyperglycemia, which occurs over a longer period of time.
There are 2 types of non-diabetic High Blood Glucose
1.Fasting Hyperglycemia
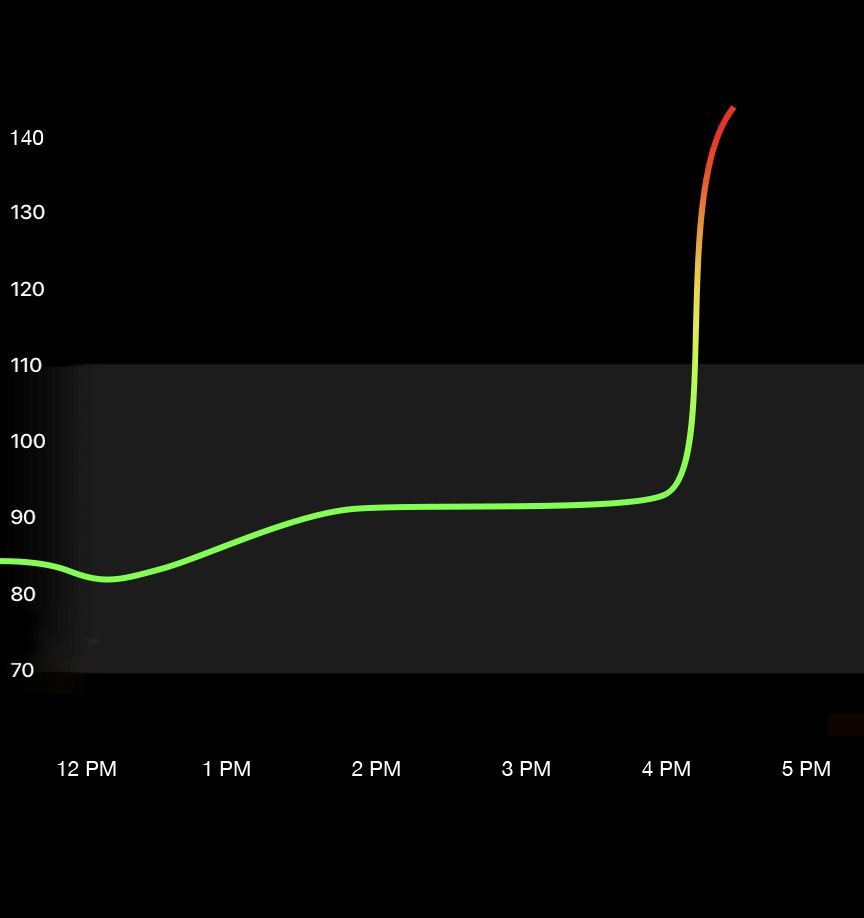
If the blood sugar level after 8 hours of fasting (or longer) is over 125–130mg/dL, it is considered fasting hyperglycemia.
2.Postprandial/reactive Hyperglycemia
If the blood sugar levels post 1-2 hours of eating is greater than 180mg/dL, it is called postprandial or reactive hyperglycemia. (1)
However, with more emerging data from the perspective of longevity (lifespan), the range of 70-110 mg/dL has been identified as a more accurate threshold. This is also something that Ultrahuman recommends within the platform which you can read more about time in range & how it affects.
Nine Symptoms of High Blood Sugar In People Without Diabetes
Even before testing for high blood sugar levels, certain common indicators can serve as a warning for postprandial/reactive hyperglycemia.
The following are the common high blood glucose symptoms
- Increased thirst and/or hunger,
- Frequent urination,
- Headache,
- Blurred vision,
- Fatigue,
- Sugar in your urine (as determined by a urine test),
- Weight loss,
- Vaginal and skin infections,
- Slow-healing cuts and sores.
Fourteen causes for high blood glucose in non-diabetics in 2022
Multiple factors leading to non-reactive hyperglycemia are:
- Cushing’s Syndrome:This syndrome is caused by excess secretion of a hormone from the anterior end of the pituitary gland called adrenocorticotropic hormone, which causes excess cortisol production from the adrenal glands. Another leading cause of Cushing’s Syndrome is pituitary adenomas or colloquially known as pituitary tumours. The cortisol levels of people with Cushing’s Syndrome spikes, increasing the risk of developing impaired glucose intolerance and hyperglycemia. Cortisol is a hormone that counteracts the effects of insulin. It blocks the uptake of glucose from the bloodstream and increases insulin resistance, thereby maintaining high glucose levels in the bloodstream. Elevated cortisol levels are also known to decrease the amount of insulin produced by the pancreas. Approximately 10% to 30% of people with Cushing’s syndrome will develop impaired glucose tolerance.
- Pancreatic disease:The pancreas is an organ located in the abdomen. Any pancreas-related disease such as pancreatitis and pancreatic cancer can cause an imbalance in the insulin levels produced by the pancreas. This improper secretion of insulin adversely impacts the blood sugar by preventing the absorption of excess glucose from the blood.
- Polycystic Ovarian Syndrome (PCOS):A hormonal disorder causing enlarged ovaries with small cysts on the outer edges.PCOS is a syndrome that commonly affects the endocrine system of women of different reproductive age groups. This condition affects roughly one in every ten women, causing infertility in women of childbearing age. Women with PCOS often have imbalanced hormones, increasing the levels of insulin in their bodies. Although there is excess insulin in the body, the absorption of glucose from the body among women with PCOS is low. As many as 30% to 40% of women who have PCOS also have insulin resistance.
- Surgery or Stress:Any physical stress on the body can cause changes, more specifically after surgery. Surgeries increase cytokines and hormones that drive the production of glucose in the liver and block the effects of insulin from removing excess glucose from the blood. Up to 30% of patients can develop stress-induced hyperglycemia after surgery, with blood glucose levels that stay elevated long after being discharged from the hospital.
- Infections:Any infection in the body leads to fatigue which physically stresses the body. This infection subsequently causes hyperglycemia as a result of increased cortisol. Cortisol, as mentioned above, blocks the ability of insulin to remove excess glucose from the bloodstream, keeping the blood sugar levels high.
Additionally, blood glucose spikes in the presence of an infection to support the kidneys, brain and red blood cells that depend on glucose for energy to aid the immune system’s response to fight off the infection.
- Medication side effects:Certain medications such as corticosteroids (medicines that closely resemble cortisol), immunosuppressants (medicines that suppress the immune system) and catecholamine vasopressors (drugs used for the specific purpose of raising blood pressure) like dopamine can release enzymes in the blood that spike blood glucose levels and disrupt the release of insulin, thereby affecting the absorption of glucose from the bloodstream.
Patients kept on IV in hospitals also have a chance of developing hyperglycemia as the nutritional fluid contains sugar to restore electrolyte balance. Therefore, this should be carefully administered in recovering patients to prevent blood sugar spikes.
- Genetics:Genes and heredity are scientific studies of how certain characteristics are transmitted from parent to child by changes in DNA sequence. There are genes in our bodies that assist us in building the molecules we need to live.
Hyperglycemia is more commonly seen in people with a family history of diabetes. Although diabetes itself can be prevented through diet and lifestyle changes, impaired insulin sensitivity can be passed on and may make the person more susceptible to reactive hyperglycemia.
- Obesity:Excess fat in the body disrupts the balance of glucose and insulin. Excess fat cells, called adipocytes, release inflammatory proteins such as interleukins and increase the body’s resistance to insulin. Adipocytes activate processes that disrupt the body’s ability to produce and release insulin when the blood sugar is high.
Adipocytes also decrease the ability to remove glucose from the bloodstream to be used as energy or stored as glycogen within the skeletal muscles. Obesity, increased lipids or fatty acids also activate pathways that impair insulin signaling within muscles.
- Lifestyle factors: Diet:Food plays an important role in the blood glucose levels of the body. An excessive amount of sugar and/or carbohydrates (including carbohydrate-containing foods) can raise blood glucose levels post meals as the food gets broken down into glucose molecules that enter the bloodstream.
In a healthy person, these glucose molecules get absorbed by the insulin released by the pancreas and then get transported to the muscles and liver for energy and storage. As the blood sugar decreases, the pancreas is signalled to stop the production of insulin, stabilising the blood glucose levels.
When blood sugar levels are continually elevated with repeated and excessive intake of carbohydrates and sugar, the excess glucose in the body stimulates the pancreas to release extra insulin. Over time, the body develops a resistance to this chronic high blood sugar as the insulin stops responding and keeps the blood glucose levels high.
Excess alcohol consumption can also affect blood sugar levels by interfering with the liver’s ability to regulate the production and release of glucose. It negatively impacts the body’s response to insulin.
- Lifestyle factors: Physical Activity – Workout:Skeletal muscles store extra glucose as glycogen for energy for later use. With low levels of physical activity, the muscles become inactive and cannot remove the excess glucose efficiently from the blood.
For instance, a notable benefit of physical activity such as walking after eating is improved blood sugar regulation. After a meal, the digestive system converts food into glucose, which subsequently floods the bloodstream. Since walking is aerobic, it allows muscles to process glucose without the body needing to produce more, and that reduces blood sugar levels. When muscles contract, they’re able to take in more glucose through muscle membranes, the heart pumps more glucose-containing blood to your muscles, and changes in chemical enzymes are involved in glucose uptake (adenosine monophosphate-activated protein kinase or AMPK) further aid the process of glucose transport. With all of these mechanisms occurring simultaneously, muscle cells utilise the glucose they need to power any activity, and our blood glucose levels drop. The lack of physical activity can hinder this process.
Non-diabetic hyperglycemia also leads to?
Nerve damage (neuropathy)
- Damage to the arteries and blood vessels, increasing the risk of heart attack and stroke
- Slow healing
- Infections by compromising your immune system
It is, therefore, crucial to take insulin or other blood-sugar regulating drugs to keep the blood sugar levels in check or consult a physician at the earliest.
Non-diabetic hyperglycemia & Metabolic Health
Metabolic health is described as having ideal levels of blood sugar, triglycerides, high-density lipoprotein (HDL) cholesterol, blood pressure and waist circumference, without using medications.
Metabolic syndrome refers to a group of conditions that simultaneously occur, elevating your risk of heart disease, stroke and type 2 diabetes. These conditions include increased blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol or triglyceride levels.
Metabolic health is impacted by the various markers mentioned above and non-diabetic hyperglycemia affects these markers as a result of various factors. It is therefore imperative to understand the factors impacting individuals with non-diabetic hyperglycemia. These factors ultimately affect metabolic health itself.
Non-diabetic hyperglycemia and stress
According to Medlineplus, stress is a feeling of emotional or physical tension. It can come from any event or thought that makes you feel frustrated, angry, or nervous.
The human body is constantly subjected to various forms of physical, mental and social stress. The sympathetic nervous system activates the body’s fight or flight response. Stress activates the sympathetic nervous system, thereby releasing a hormone called cortisol. Cortisol plays a crucial role in regulating blood pressure and blood sugar levels. When the body is subjected to stressors, cortisol causes increased glucose levels for an immediate energy source, inhibiting sensitivity to insulin production. Over time, with chronic stress and chronically elevated glucose levels, the pancreas (responsible for insulin production) loses the ability to respond to a high glucose stimulus, causing a reduction in the activity of insulin.
Chronic stress can place a high amount of load on the body during stressful situations and can indicate to the metabolic system to meet the requirements of the increased demand. This constant load can impact an individual’s immunity and predispose them to contract diseases that are caused by insulin resistance and alteration in glucose metabolism such as metabolic syndrome and diabetes. Chronic stressors also impair the function of insulin (causing Insulin resistance) affecting postprandial (after a meal) glucose metabolism.
Acute psychological and physiological stress stimulates the HPA axis. The Hypothalamic Pituitary Adrenal (HPA) axis represents the interaction between the hypothalamus, pituitary gland, and adrenal glands, playing an important role in the stress response. It interferes with carbohydrate metabolism by increasing glucose availability and insulin levels. While the effects of acute stress are normal and given enough time to recover can even be beneficial, chronic stress can be a problem. Over time, chronic stress can lead to insulin insensitivity, altered glucose and insulin metabolism, which increases an individual’s propensity to develop type 2 diabetes. Constant exposure to chronic stress can lead to increased consumption of food, impacting metabolic health and weight gain. In due course, this can lead to glucose intolerance and cardiovascular diseases.
Non-diabetic hyperglycemia and heart health
Heart health and non-diabetic hyperglycemia are closely related. Research suggests that blood glucose level is a risk marker for cardiovascular disease (CVD) among apparently healthy individuals without diabetes. While stress can trigger high blood sugar levels and low insulin, it can, as a byproduct, cause cardiovascular disease too. This CVD occurs as a result of high blood sugar levels.
Elevated blood glucose levels lead to high levels of inflammation in the body. This then causes damage to the artery linings and forms plaque in the arteries. If there is a buildup of plaque in one of the heart’s arteries, it completely obstructs blood flow and leads to a heart attack, also known as acute myocardial infarction. (8) If one of the arteries to the brain gets clogged, it will cause a stroke. People with high blood glucose levels also have a proclivity for dense LDL cholesterol that finds easy access into blood vessel walls and creates plaque. These walls then become thicker and are conducive to high blood pressure, leading to further, more serious diseases in the heart. (9)
Over time, such pockets of glucose hold the potential to evolve into conditions like diabetes and cause various heart-related conditions.
Insulin resistance can also lead to weight gain correlated with heart strain. This strain can further cause heart failure because the heart is unable to pump blood as effectively as it should. Additionally, fluid can also build up in the legs (leading to peripheral arterial disease) and lungs, making breathing difficult.
Non-diabetic hyperglycemia and non-alcoholic fatty liver disease (NAFLD)
People with fatty liver disease are often predisposed to insulin resistance. The cells in their body produce insulin but can’t utilise it efficiently and are resistant to its influence. Therefore, the blood glucose levels tend to remain high and the liver turns it into fat.
When triglycerides accumulate in the liver, it can lead to increased gluconeogenesis, decreased glycogen synthesis, and inhibition of insulin signalling.
High-density lipoprotein (HDL), a cardiovascular disease protective marker, is found to decrease in patients with fatty liver disease.
Studies suggest that the accumulation of belly fat may symbolise an important underlying mechanism for the correlation between liver enzymes and hypertension.
Non-alcoholic fatty liver disease (NAFLD) has more layered causal elements. Currently, research indicates that high levels of blood pressure and cholesterol are factors, as is obesity.
Non-diabetic hyperglycemia and serum lipoprotein
Lipoproteins are substances made of protein and fat that carry cholesterol through the bloodstream. Serum lipoprotein is the total of LDL, HDL, and triglycerides. Research demonstrates that serum lipoprotein can provide a simple means of identifying insulin resistance and can be used as markers of insulin resistance and cardiovascular diseases risk in adult non-diabetic patients. Non-diabetic metabolic syndrome patients show higher levels of triglycerides post a meal than patients without metabolic syndrome.
Non-diabetic hyperglycemia and obesity
Research has found that morbidly obese individuals who are non-diabetic or prediabetic have unstable blood glucose levels as compared to people with a normal BMI. Therefore, an elevated glucose level may be an important risk factor in the increased cardiovascular disease seen in obese and morbidly obese people who are not diabetic.
Excess fat cells (adipocytes) disrupt the balance of glucose and insulin. They release inflammatory proteins and increase the body’s resistance to insulin. Adipocytes activate processes that disrupt the body’s ability to produce and release insulin when the blood sugar is high.
Adipocytes also decrease the ability to remove glucose from the bloodstream to be used as energy or stored as glycogen within the skeletal muscles. Obesity, increased lipids or fatty acids also activate pathways that impair insulin signalling within muscles.
Fifteen ways to prevent high blood glucose levels (Hyperglycemia)
Multiple lifestyle changes that can be made to treat and manage Non-diabetic hyperglycemia in 2022 are:
1.Exercise regularly:Regular exercise of at least 30 mins a day, five days a week, helps lower blood sugar levels, especially when the levels are high. Skeletal muscles store extra glucose as glycogen for energy for later use. When you do moderate exercise, like walking, it makes your heartbeat a little faster and your breath a little harder. Your muscles use more blood glucose thereby lowering your blood sugar levels over time. It also makes the insulin in your body work better. The benefits last for hours after the workout. Over time, it can also stabilise your blood sugar. For example, strength training can expand the storage capacity for blood sugar in the muscle and improve insulin sensitivity. Children with reactive hyperglycemia are recommended to get at least 60 minutes of physical activity each day.
2.Maintain a healthy weight:Maintaining a healthy weight can help prevent irregular insulin production and can absorb excess glucose from the blood optimally.
3.Limit alcohol intake:The liver is a vital organ in regulating blood sugar levels. Alcohol initially leads to a spike in glucose level, which is generally metabolised speedily. Therefore, limiting alcohol intake in both men and women can prevent reactive hyperglycemia.
4.Avoid smoking:Cigarettes, E-cigarettes and smokeless tobacco contain nicotine and other chemicals that cause lung damage. Nicotine changes chemical processes in your cells so they don’t respond to insulin. The chemical also alters the way your body can use glucose and this leads to insulin resistance.
5.Eat earlier in the day:Consuming all your days worth of calories in a relatively shorter window and abstaining from eating beyond that timing helps keep blood glucose levels in check. This is called ‘time-restricted feeding’. A study conducted on non-diabetic overweight people showed that if time-restricted feeding is practiced for 4 days or more, the person will see reduced fasting glucose levels, fasting insulin levels and mean glucose levels.
The timing of food intake makes a big difference in the level of glucose in our blood. Naturally, bodies are more resistant to insulin at night so even if the same food is eaten at night, the morning food will have a smaller glucose spike than the same food eaten at night.
6.Explore intermittent fasting:Intermittent fasting means restricting food intake for long periods, usually for 24 hours or more. If a longer gap is left between meals, insulin levels in the body reduce and fat cells can then release their stored sugar, to be used as energy. As a by-product, the body will also shed weight, leading to a further reduction in insulin levels.
Intermittent fasting is thought to increase the expression of genes, hormonal pathways, and cellular physiology that improve metabolic fitness and insulin sensitivity.
7.Avoid refined sugar and foods:This is a simple and highly effective concept. To optimise glucose levels, avoid food that was made in a factory, is packaged or doesn’t retain its original form.
Added sugars, refined grains and fruits (such as juices) are at the top of the list of foods that need to be avoided to maintain a healthy glucose level. Naturally occurring sugars in their whole food form, like sugars in fresh fruit, are generally going to have less of an effect on glucose levels, as they will be surrounded by unprocessed fibres and other nutrients, making them slower to be digested.
8.Include lots of fiber:High fiber diets improve glycemic control in individuals with insulin resistance. Research shows that higher fiber content leads to better glucose-related metrics. Fiber sources such as beans, nuts, seeds, vegetables, fruits, or whole grains must be included in every meal.
9.Get adequate sleep:Sleep is critical for glucose regulation and metabolic fitness. Sleeping for just 4 hours per night, five days a week has been shown to notably decrease glycemic control and thereby metabolic fitness. Studies show that this level of sleep deprivation leads to a spike in blood glucose in response to some foods and a 40% lower rate of glucose being flushed out of the system compared to people who get 12 hours of sleep per night.
Studies, however, also indicate that blood glucose levels can be brought into control or reversed in as little as two days of getting proper sleep.
10.Use Vinegar to blunt spikes:Vinegar is commonly known to lower glucose levels when taken before or with a meal. Studies show that 1 ounce of white vinegar if consumed with a carbohydrate-rich meal reduces postprandial responses of blood glucose and insulin. Vinegar also increases satiety.
11.Use fat and protein to your advantage:Preloading meals with fat or protein can minimise the quick absorption of glucose into the bloodstream. A study found that when people who are non-diabetic and insulin resistant consumed 23 grams of protein and 17 grams of fat 25-30 minutes before eating their carbohydrates, there was a significant decrease in their post-meal glucose elevation.
Similarly, eating fat alone in conjunction with a carbohydrate load will decrease the post-meal glucose spike. Research shows that eating 3 ounces of almonds with a meal of white bread leads to significantly lower post-meal glucose spikes than when white bread is eaten alone. Similar trends were seen when participants were served 1 and 2 ounces of almonds, but the biggest effects were seen with 3 ounces of almonds (40g of fat).
12.Control stress:Stress releases cortisol in the blood and cortisol plays a crucial role in regulating blood pressure and blood sugar levels. When the body is subjected to stressors, cortisol causes increased glucose levels for an immediate energy source, inhibiting sensitivity to insulin production.
Fortunately, stress can be managed with a few simple and effective techniques that reduce glucose levels. For instance, a study conducted on people with insulin resistance showed that when they engaged in just 20 minutes of diaphragmatic breathing, their blood sugar post-meals reduced significantly at the 9th week of the study. Do you know how blood glucose is connected with the breathing process?
Additionally, six months of meditation done twice a week in individuals with heart disease has been shown to result in a significant decrease in fasting blood sugar, post-meal blood sugar and hemoglobin a1c.
13.Limit intake of saturated fats:Eating large amounts of saturated fat has been shown to acutely decrease whole-body insulin sensitivity by about 25%. Saturated fats include fatty cuts of beef, pork, lamb, dark chicken meat, poultry skin, dairy foods (milk, butter, cheese), tropical oils like coconut and palm, and margarine. To optimise insulin sensitivity, emphasising unsaturated fats like nuts, seeds, olives, olive oil, avocado, fish, soybeans and tofu appear to be a better bet.
14.Don’t chug water with meals: Multiple studies have shown that drinking a large amount of water with a meal will increase the glucose and insulin peak after a meal, likely because the fluid load speeds entry of food into the small intestines for rapid glucose absorption. Although good hydration overall is an important part of metabolic health, try to avoid chugging it.
15.Add cinnamon to food:Cinnamon has been found to improve insulin signalling and glycemic control through several potential mechanisms in non-diabetic hyperglycemic individuals. Based on research, cinnamon may be a helpful adjunct in the quest for improved metabolic fitness. Given that high post-meal glucose spikes are associated with worse health outcomes, cinnamon may be an effective way to blunt these surges.
Conclusion
The hormone insulin controls the metabolism of carbohydrates, fats and protein by advancing the absorption of glucose from the blood into the liver, fat and skeletal muscle cells. Glycogenolysis and gluconeogenesis, the sources of circulating glucose, are partially under the influence of glucagon, another hormone produced in the pancreas. A rise in blood glucose levels in the body is called hyperglycemia. A rise in blood glucose levels in non-diabetic people is called non-diabetic or reactive hyperglycemia. Elevated glucose levels are caused by various factors such as stress, obesity and ingesting sugars that break down quickly in the body. Non-reactive hyperglycemia can prevent healing and amplify the risk of heart diseases among other detrimental factors. Exercise, limiting sugar intake, avoiding smoking and eating earlier in the day are some ways to manage non-diabetic hyperglycemia.
Disclaimer: The contents of this article are for general information and educational purposes only. It neither provides any medical advice nor intends to substitute professional medical opinion on the treatment, diagnosis, prevention or alleviation of any disease, disorder or disability. Always consult with your doctor or qualified healthcare professional about your health condition and/or concerns and before undertaking a new health care regimen including making any dietary or lifestyle changes.
References
- https://www.endocrineweb.com/conditions/hyperglycemia/hyperglycemia-when-your-blood-glucose-level-goes-too-high
- https://my.clevelandclinic.org/health/diseases/9815-hyperglycemia-high-blood-sugar
- https://www.verywellhealth.com/what-is-the-pancreas-3289656
- https://link.springer.com/article/10.1007%2Fs11102-013-0483-3
- https://link.springer.com/article/10.1007%2Fs11102-013-0483-3